Abstract
Reaction of benzyl alcohols with Oxone and sodium bromide in aqueous acetonitrile gave the corresponding benzaldehydes in excellent yields. However, electron-rich benzyl alcohols afforded ring bromination products via bromodecarbonylation of the resulting benzaldehydes.
The selective oxidation of benzylic alcohols to benzaldehydes is a transformation of considerable importance in organic synthesis. Whilst numerous reagents have been developed to effect this process, many of them use greater than stoichiometric quantities of toxic heavy metals or co-oxidants which severely handicap their applicability to large scale industrial processes1,2. Also, the oxidation of organic compounds by hypohalite salts or halogen is well known method in organic synthesis3. Especially, oxidation of primary alcohols to aldehydes with hydrogen peroxide using methyltrioxorhenium and bromide ions as cocatalysts4, and with oxoammonium salt and bromide ions5 have been described.
Recent reports have dealt with the use of a triple salt of potassium peroxymonosulfate, potassium hydrogen sulfate, and potassium sulfate, which is commercially available as Oxone (2KHSO5·KHSO4·K2SO4), can be used for the oxidation of alkenes6, arenes7, amines8, imine9, sulfides10, selenides11, α-amino acids12, and acetals13. Also, there are reports in the literature, where Oxone is a useful oxidation reagent of alcohols and aldehydes. Examples include the conversions of 2-propanol to acetone6, ethanol to ethyl acetate6, and benzaldehyde to benzoic acid6,14. Another example is the oxidation of secondary alcohols to ketones in the presence of wet-aluminium oxide in aprotic solvents15. Also, Bolm and co-workers have demonstrated that the combination of TEMPO/Oxone/Bu4NBr is an effective system for the oxidation of alcohols to aldehydes and ketones, including benzylic ones16. Moreover, the use of Oxone and aqueous sodium halides was conducted as a convenient halogenating reagent to achieve oxidation of α,β-enones17, bromination of pyrimidines18, and halogenation of toluene6.
In previous paper, we have shown that sodium bromide combined with Oxone serves as effective bromodecarboxylation reagent of various cinnamic acids19 and halogenation of aromatic methyl ketones20. In the course of our study to extend the scope of the Oxone/NaBr reagent in organic synthesis, we have found that this reagent facilitates the oxidation of benzylic alcohols to benzaldehydes satisfactorily.
Optimization of the reaction conditions revealed that simple stirring a solution of benzyl alcohol (1 eqv), Oxone (1 eqv) and sodium bromide (2 eqv) in a 1:1 mixture of CH3CN/H2O effected the formation of benzaldehyde in 87% isolated yield within 3 h. However, in the absence of sodium bromide, the reaction did not proceed at all in 24 h at r.t. Further studies showed that this oxidation method could be applied to a wide range of benzylic alcohols and representative primary and secondary alcohols as shown in Table 1. They are all known compounds and are identified by their IR, 1H NMR and mass spectral data.
Scheme 1
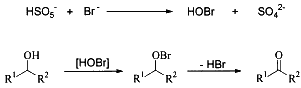
Table 1
Oxidation of Alcohols with Oxone and NaBr
[Table missing. If found, please email it to me]
A plausible mechanism of the oxidation is shown in Scheme 1 based on literatures. The oxidation of bromide ion by peroxymonosulfate ion would give the hypobromous acid16,21 and subsequent oxidation of alcohols affords aldehydes and ketones.
The presence of electron-donating groups in the aromatic ring has little influence on the oxidation rates but these are markedly lowered by introducing a strong electron-acceptor group. Thus, p-nitrobenzyl alcohol was oxidized to aldehyde in only 20% yield over 24 h. Also, the reaction was unsuccessful for electron-rich arene such as p-methoxybenzyl alcohol, which presumably suffered complications due to competing bromodecarbonylation of the resulting p-anisaldehyde which was accompanied by the formation of 4-bromoanisole (44%) and 2,4-dibromoanisole (14%)22. Similarly, p-acetamidobenzyl alcohol gave bromodecarbonylation products, 4-bromoacetanilide (63%) and 2,4-dibromoacetanilide (20%)22. In the oxidation of 1-phenyl-1,2-ethanediol, the secondary benzylic alcoholic function was oxidized with high selectivity to form 2-hydroxyacetophenone in 99% yield. The oxidation of secondary alcohols afforded the corresponding ketones in excellent yields. But, primary alcohol such as 1-pentanol was converted mainly into the dimeric ester, pentyl valerate, presumably via hemiacetal intermediate23.
In conclusion, we developed a simple oxidation method of benzyl alcohols to benzaldehydes with Oxone/NaBr in aqueous acetonitrile under the mild conditions. This method provides an alternative, facile preparation of benzaldehydes, since Oxone and sodium bromide are cheap, nontoxic, stable, and easy to handle.
Experimental
- 1: Liquid (Lit.24 bp 178?C)
- 2: mp 47?49?C (Lit.24 47.5?C)
- 3: Liquid (Lit.24 bp 204?205?C)
- 4: mp 103?105?C (Lit.24 106?C)
- 5a: Liquid (Lit.24 bp 249.5?C)
- 5b: Liquid (Lit.24 bp 215?C)
- 5c: mp 61?62?C (Lit.25 61?63?C)
- 6a: mp 165?167?C (Lit.24 168?C)
- 6b: mp 142?143?C (Lit.26 144.7?C)
- 7: mp 79?81?C (petroleum ether)
(Lit.24 90?C) - 8: Liquid (Lit.24 bp 202.6?C)
- 9: Liquid (Lit.24 bp. 155.6?C)
- 10a: Liquid (Lit.24 bp. 203.7?C)
- 10b: Liquid (Lit.24 bp. 186?C)
Melting points were determined in open capillaries with an Electrothermal melting point apparatus and are uncorrected. Progress of reactions were followed by TLC using silica gel with fluorescent indicator coated on aluminium sheets.
General Procedure for the Oxidation of
Alcohols with Oxone and Sodium Bromide
To a stirred solutions of alcohols (3 mmol) in aqueous CH3CN (30 mL, 1:1 by volume) was added NaBr (0.62 g, 6 mmol) and Oxone (1.84 g, 3 mmol). Reactions were continuously monitored by thin-layer chromatography and stirred at r.t. for the time indicated in Table 1. The reaction mixture was quenched with aqueous sodium thiosulfate, and extracted with ether (3?30 mL). The combined organic layers were washed with water, dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was chromatographed on a silica gel column and eluted with hexane?EtOAc 10:1 to give the products (Table 1).