Abstract
Previous studies on the khat plant (Catha edulis) illustrated the importance of using freshly harvested young shoots and leaves such that cathinone, the principal active component and Schedule I controlled drug contained within the plant, could be suitably isolated and identified. Upon drying and storage of the cut plant material, cathinone readily converts to the reduced product, cathine, which necessitates rapid extraction and chemical analysis for cathinone identification. This study demonstrates that by air drying the young khat shoots at ambient temperature, cathinone may be detected in khat samples that have been harvested for more than 10 days. Refrigeration for two weeks and freezing for one month of the khat samples also yield identifiable levels of cathinone. Cathinone and cathine are both specifically determined and differentiated by vapor phase infrared detection, which is the method of choice in relation to mass spectrometry.
Note by Rhodium:
This document was prepared from a bad photocopy, and unreadable words has been replaced by [...] below.
If you are able to aquire a better copy of this article, please email it (or at least the missing words) to me.
The chewing of khat, which typically consists of the young leaves and stems of the Catha edulis plant, for its stimulant effects has in the past been largely confined to its area of cultivation, namely, East Africa and the Arab Peninsula. Historically, cathine [(+)-norpseudoephedrine] (Fig. 1), was erroneously identified to be the active compound in khat which was responsible for the observed pharmacological effects because the investigations were based on dried or old plant samples. However, discrepancies in drug potency signaled to researchers that another compound was accountable for the effects. Using fresh young leaves or otherwise well-preserved khat samples, cathinone (2-amino-1-phenyl-1-propanone) (Fig. 2), was finally isolated and identified as the principal alkaloid1-8. Cathinone is estimated to be one-third as potent as amphetamine and ten times more potent than cathine9, and it has been found that during the maturation of the leaves or decomposition of the plant through drying and storage, cathinone contained within the plant is enzymatically converted to cathine5.
Fig. 1-2
Cathine (left) and Cathinone (right)
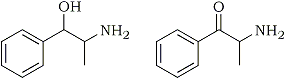
Effective February 1993, cathinone was placed into Schedule I of the Controlled Substances Act10. Khat is a Schedule I substance when cathinone is present. Cathine is ruled as a Schedule IV substance11. When the plant no longer contains detectable levels of cathinone, as in older leaf samples, but contains cathine khat is classified as a Schedule IV substance. This causes a logical problem with cathinone detection since fresh khat leaves (or an otherwise well-preserved sample) are needed for the analysis, an order for the sentence of a Schedule I drug to be invoked.
Fresh leaves are traditionally chewed as the cut khat loses its potency about 3 to 4 days after harvesting2. Despite the short period of drug effectiveness, the availability of rapid air freight service has resulted in the appearance of khat in non-origin countries, for example, United States, Italy, and Great Britain. Although it is now recognized that the rapid extraction and analysis of fresh khat samples is needed, the time frame required for cathinone detection has not yet been adequately addressed. Further, the fo[...] of past khat studies relied upon using freshly picked khat samples, deep-freeze and freeze-dried preservation techniques, and temporary dry ice storage so that cathinone detection would be inst[...]2,7,9,12,13. For law enforcement purposes, such specialized preservation techniques are not generally feasible or readily available at seizure sites. More practical means of sample storage and preservation were investigated in this study so that reasonable guidelines and expectations for cathinone detection in seized khat drug evidence could be made available. In addition to this, the use of vapor phase infrared detection (IRD) and mass spectrometry (MS) were evaluated for their specificity in cathinone and cathine identification.
Experimental
For this study, freshly picked khat shoots were sent from a domestically grown source to the Drug Enforcement Administration (DEA) Western Laboratory via express mail in a padded envelope, packed with shredded paper. The plant specimen was portioned into smaller parcels. The bulk of the sample was left to dry in a brown paper bag that was kept at ambient temperature and protected from light. Smaller sample portions were heat-sealed in evidence envelopes (that is, 0.0045-inch thick polyester barrier and placed in the laboratory refrigerator (-2?C) and freezer 11?C). For the time study, a simple and rapid method to extract the compounds of interest was devised based on standard alkaloid extraction techniques2. The procedure is described as follows.
- Extraction
Approximately 5 to 6 g of plant material was cut into small pieces, dimensions of approximately 0.5 cm squares. The prepared sample was mixed with 15-20 mL of methanol, then sonicated for 15 min with intermittent shaking and stirring. - Concentration
The green methanolic extract was decanted into a breaker which was then condensed to near dryness (<1 mL) using a stream of air. - Cleanup
A very dilute solution of H2SO4 (approx. 0.02 N) was used to resuspend and acidify the plant residue. The acidified solution acquired a reddish hue. A chloroform extraction was performed to remove the neutral organic compounds as well as the remaining plant solids. - Base Extraction
A small amount of a saturated sodium bicarbonate (NaHCO3) solution was added to the aqueous solution to basify the extract. The pH change resulted in a light green solution. Methylene chloride was used to extract cathinone and cathine. A stream of air was used to reduce the extract to a minimal volume amount. (As a preventive measure, a solvent exchange to hexane is done to avoid degradation of the IRD light pipe.) Instrumental analysis was performed immediately after the base extraction8.
Vapor Phase Infrared Spectroscopy
The vapor phase infrared spectra were obtained on a Hewlett-Packard Model 5890 Series II Gas Chromatograph, using a 12 m by 0.32 mm HP-5 (0.52 µm loading) capillary column and a temperature program of 70?C for 1 min, 15?C/min to 270?C, with a final temperature hold for 5 min, equipped with a Hewlett-Packard Model 5965B Infrared Detector.
Mass Spectrometry
Mass spectra were obtained on a Hewlett-Packard Model 5890 Series II Gas Chromatograph, using a 12 m by 0.22 mm HP-1 [...]µm loading) capillary column and a temperature program [...]0?C for 2 min, 15?C/min to 300?C, with a final temperature hold [...]5 min, equipped with a Hewlett-Packard 5970 Mass Selective Detector (electron impact mode).
Thin Layer Chromatography
The plant extract was spotted directly onto a precoated 5 by 10 cm silica gel 60 (Kieselgel F254) plate. Cathinone and cathine drug standards (cathinone hydrochloride supplied by RSA Corporation, cathine base and cathine hydrochloride provided by DEA's National Testing Laboratory) which were dissolved in methanol [...] also applied. The plate was developed in ethyl acetate:methanol:aqueous ammonia (85:10:5), then viewed under an ultraviolet lamp [...] nm)[...]2,7,8,12,14. The spots were visualized using a 0.5% ninhydrin solution, and then the plate was heated using a heat gun. Cathine appeared purple, while cathinone was a burnt orange [...] moving spot). The Rf values obtained for cathinone and cathine were 0.46 and 0.25, respectively.
Results
Dried Samples Stored at Room Temperature
[...] Day 1 as the day the khat material was harvested, 5 g leaves and stems were removed from the paper bag on a daily [...] and analyzed. Cathinone was detected as the major component [...] the Day 2 run of the IRD total response chromatogram [...] through Day 7 (Fig. 3). None or only a trace amount of [...] was detectable. On Day 5, cathine became detectable, in [...] to cathinone. By Day 11, the cathinone IRD peak height [...] TRC was twice or equivalent to the cathine peak, which made a positive identification for cathinone difficult, but still possible, due to the poor chromatographic separation. As the plant dried, more leaves were needed to yield the 5 g sample size. Analyses [...] longer time periods were not performed since there was insufficient [...] air-dried sample. The IRD detection of cathinone after 10 days of air drying is consistent with an earlier work which used thin layer chromatography (TLC) to verify the presence of cathi[...] collected khat samples that were first stored in thermoflasks containing dry ice for 3-4 days, then later allowed to air dry for 7 to 10 days12.
The IRD spectra for cathinone and cathine isolated from the Catha edulis cuttings are shown in Figs. 4a,b and the mass spectra for cathinone and cathine standards are provided in Figs. 5a,b. Previous works have described using high-pressure liquid chromatography, gas chromatography, infrared spectroscopy, MS and TLC to identify cathinone and cathine. The mass spectra for the cathinone and cathine standards (Fig. 5a,b) are very similar, with an identical base peak at m/e 44 for both compounds. Some groups have used acetylation to better identify the two components by MS8. However, the IRD spectra for cathinone and cathine are unambiguous and unique (Fig. 4a,b).
Refrigerated Samples
A 3.5 g khat sample that was stored in a refrigerator for two weeks, exhibited cathinone and cathine (Fig. 6).
Frozen Samples
Samples stored in the freezer for 34 days and 48 days before showed cathinone and cathine, with the cathine levels in high concentration in the latter sample (Figs. 7a,b).
Actual Khat Exhibit
While this study was in progress, an actual khat exhibit was submitted to the Western Laboratory within four days of a U.S. Customs import seizure. Although the sample was sent by overnight delivery and analyzed on the day of receipt, the exhibit had undergone significant spoilage as evidenced by a foul smell and a disproportionately large amount of wet, brown leaves. There have been a number of [...] seizures submitted to several DEA field laboratories where the plant shoots were bundled and wrapped in large plant leaves. In this particular case, the submitted plant exhibit was packaged in plastic bags rather than in leaves which severely restricted air flow around the plant cuttings. Although approx. 50% of the leaves still appeared to be in a fresh condition and were selectively used for the analysis, cathinone was fairly difficult to detect since its IRD peak height was only one-third that for cathine.
Recommendations for submitting khat samples to the laboratory include:
- Sending a representative sampling of the exhibit in [...] paper bags
- Packing the bags in sturdy cardboard boxes (which should then be properly sealed)
- Providing advance notice of the khat shipment to the laboratory
- Sending the shipment by overnight delivery
- Storing the plant cuttings in a cool area until the analysis is conducted
[...] Findings
Work by the DEA Northeast Laboratory has found that cathinone concentrated in the leaves rather than in the stems which is consistent with previous studies12,13. This present work supports earlier findings2,5,9 that once the plant cuttings are harvested, the cathinone concentration within the plant gradually decreases with the concomitant formation of cathine. However, once cathinone is extracted from the khat leaves, the reduction process from cathinone to cathine ceases to occur.
Fig. 8
3,6-Dimethyl-2,5-Diphenylpyrazine

An undesired side reaction which develops during the cathinone isolation step is the oxidative dimerization of cathinone (3,6-dimethyl-2,5-diphenylpyrazine)2,7,8, and which significantly decreases the amount of cathinone detected. [...] rapid workup and analysis are recommended once the extraction process is completed to facilitate the detection of cathinone8. Although the results presented in this study are based on several khat plants, it should be noted that cathinone levels in the plant can be highly variable; depending on factors such as species, plant maturity, [...] and quality9.
Conclusions
This study provides conclusive results that cathinone can be extracted from harvested khat (Catha edulis) despite the elapse of almost two weeks to a month, when the plant samples do not visibly appear to be in their original freshly cut condition. Through proper sample handling, the sample holding time for khat plant material may be significantly extended while at the same time, allow for legal due process of a Schedule I drug, cathinone, rather than the Schedule IV drug, cathine.
The use of vapor phase infrared spectroscopy to identify cathinone and cathinone provides unambiguous and unequivocable spectra relative to that obtained by mass spectrometry.
- Fig. 1: Cathine
- Fig. 2: Cathinone
- Fig. 3: IRD total response chromatogram for plant extract
- Fig. 4a: IRD spectrum of cathinone from khat
- Fig. 4b: IRD spectrum of cathine from khat
- Fig. 5a-b: Mass spectrum of cathinone and cathine standard
- Fig. 6: IRD total response chromatogram of khat (refrigerated 2 weeks)
- Fig. 7a-b: IRD total response chromatogram of khat (frozen 34 and 48 days)
- Fig. 8: 3,6-dimethyl-2,5-Diphenylpyrazine
- Fig. 9: Mass spectrum of cathinone dimer