Abstract
A fatality following ingestion of diazepam and 4,5-dihydro-4-methyl-5-phenyl-2-oxazolamine, a cyclic derivative of phenylpropanolamine known as U4EuH or 4-methylaminorex, is described. Solid dosage samples of U4EuH were analyzed using gas chromatography, ultraviolet and infrared spectroscopy, nuclear magnetic resonance, and mass spectrometry. Physiological fluids were analyzed quantitatively by gas chromatography and qualitatively by gas chromatography-mass spectrometry. Concentrations of 4,5-dihydro-4-methyl-5-phenyl-2-oxazolamine were: in blood 21.3 mg/L; in urine 12.3 mg/L. Diazepam concentration in blood was 0.8 mg/L.
Case History
A 37-year-old white male with a history of clandestine chemical manufacturing was found dead, lying face down on a bed in a hotel room. Discovered on his person was a plastic bag containing white powder (0.1 g) labelled "4-methyl aminorex free base, not for injection, for sacramental use only."
Analysis of the white powder and toxicological testing of the subject's blood and urine are described below. The autopsy results were respiratory center paralysis, visceral congestion, cerebral edema, and pulmonary edema. An accidental overdose was considered the cause of death.
A cyclic derivative of phenylpropanolamine known on the street as U4Euh or 4-methylaminorex has been identified in several solid dosage drug cases in central and northeast Florida. This chemical, cis-(?)-4,5-dihydro-4-methyl-5-phenyl-2-oxazolamine (CAS number 29493-77-4), was first reported in 19631 as a potential anorectic agent. Its effects have been reported to be similar in several respects to those of amphetamine1-3.
At this time, U4Euh is not controlled under Federal or Florida statutes. It is readily synthesized from phenylpropanolamine, an easily obtained noncontrolled substance1. These factors give U4Euh a high abuse potential by illegal drug users looking for a legal alternative. This report covers the synthesis, analysis, and finally the involvement of U4Euh in a death.
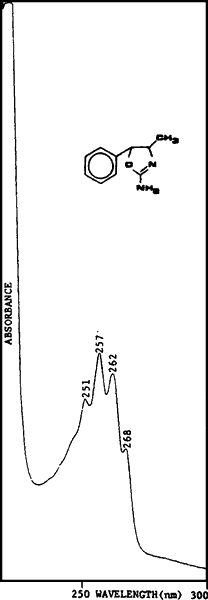
Fig. 1.
Ultraviolet spectrum of
4,5-dihydro-4-methyl-5-phenyl-
2-oxazolamine in 0.1N H2SO4
Materials and Methods
Synthesis of cis-(?)-4,5-Dihydro-4-Methyl-5-Phenyl-2-Oxazolamine
The compound was synthesized from norephedrine using the procedure of Poos et al.1
Ultraviolet Spectrophotometry
Ultraviolet spectra of 0.1N sulfuric acid (H2SO4) solutions of solid dosage samples were obtained on a Perkin-Elmer Model 200 spectrophotometer.
Infrared Spectrophotometry
Infrared spectra of KBr discs of solid dosage samples were obtained using a Beckman Microlab 620 Mx high resolution instrument.
Nuclear Magnetic Resonance (NMR)
Solid dosage samples were analyzed using a Varian EM-360 60-MHz 1H-NMR with deuterochloroform (CDCl3) with 1% tetramethylsilane (TMS) as the solvent.
Gas Chromatography
Solid dosage and biological extracts were analyzed using a Hewlett Packard 5890 gas chromatograph equipped with a capillary column operated in the split mode (100:1) and a flame ionization detector. A 12-m methyl silicone narrow bore column using helium carrier was utilized along with temperature programming from 100-300?C at 25?C/min.
Gas Chromatography-Mass Spectrometry
A Hewlett Packard 5970 mass selective detector interfaced with a Hewlett Packard 5890 gas chromatograph was used to analyze solid dosage and biological samples. The 10-m methyl silicone narrow bore capillary column was operated in the split mode (50:1). The mass spectrometer was operated in the electron impact mode (70 eV).
Blood and Urine Extraction Procedure
Samples (3 mL) of blood or urine combined with 0.2 g of sodium bicarbonate:sodium carbonate (3:1) were extracted with 10 mL of hexane:ethyl acetate (70:30). The organic layer, which was removed, was extracted with 3 mL of 1N hydrochloric acid (HCl). The pH of the aqueous layer was adjusted to pH = 10 with sodium carbonate and extracted with 100 µL of chloroform. Aliquots of the chloroform layer were then analyzed. Methaqualone was used as the internal standard for quantitations.

Fig. 2.
Infrared spectrum of 4,5-dihydro-4-methyl-5-phenyl-2-oxazolamine in KBr.
Results and Discussion
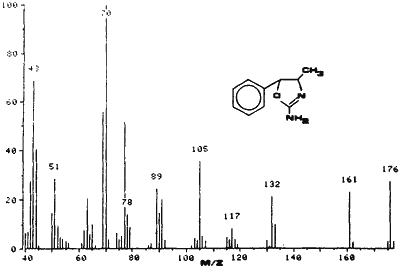
Fig. 3.
EI-MS of 4,5-Dihydro-4-methyl-5-phenyl-2-oxazolamine
A sample of cis-(?)-4,5,dihydro-4-methyl- 5-phenyl-2-oxazolamine was synthesized for use as a standard. The synthetic sample and the powder from the case were compared using various testing techniques and proved to be identical. Analysis of U4Euh with ultraviolet spectrophotometry resulted in a benzenoid absorption pattern (Fig. 1). A retention index of 1600 methylene units was obtained for this compound utilizing a methyl silicone narrow bore capillary column and temperature programming. The infrared spectrum (Fig. 2) is interesting in that it contains a carbonyl-like absorption at 1700 cm-1 as a result of the imine bond. The electron impact mass and NMR spectra (Figs. 3 and 4) are also useful for the identification of this compound. The base peak (m/z = 70) in the mass spectrum is not a normally encountered ion and consequently aids greatly in the identification.
The solid dosage form of U4Euh has been identified in approximately 15 cases in central and northeast Florida. It has been represented by dealers as both cocaine and methamphetamine. The case reported here is the first time it has been reported as being involved with a fatality.
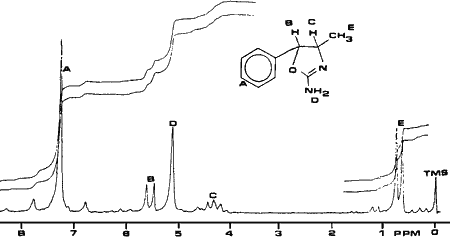
Fig. 4.
1H-NMR (CDCl3) spectrum 4,5-Dihydro-4-methyl-5-phenyl-2-oxazolamine
Using methaqualone for the internal standard and gas chromatography for the analysis, 21.3 and 0.8 mg/L of U4Euh and diazepam were found in the blood of the victim, respectively. Only U4Euh, 12.3 mg/L, was found in the urine.
A study by Dinovo et al.4 of diazepam in drug related deaths found mean blood concentrations to be 18 mg/L. The 0.8 mg/L of diazepam which is a therapeutic concentration5 appears not to be the cause of death. There is no published data on human toxicity of U4Euh. There have been reports of similarities in activities of U4Euh and amphetamine1-3. In a study of amphetamine related fatalities, average blood and urine levels of 8.6 and 237 mg/L were reported, respectively5. The high blood level of U4Euh (21.3 mg/L) in this case appears to be a significant factor in this fatality.
Conclusion
Data have been presented from various chemical examinations used in identifying 4,5-dihydro-4-methyl-5-phenyl-2-oxazolamine. This chemical, also known as U4Euh and 4-methylaminorex, is readily synthesized from phenylpropanolamine and has activity similar to amphetamine1-3. The blood and urine levels from the first reported fatality associated with this chemical were determined to be 21.3 and 12.3 mg/L, respectively.